PulseFlow: fighting the war against diabetic foot ulcers
James Faulkner: Thanks for taking the time to speak with Master Investor. Can you begin by giving our readers a bit of background information on PulseFlow and its recent history?
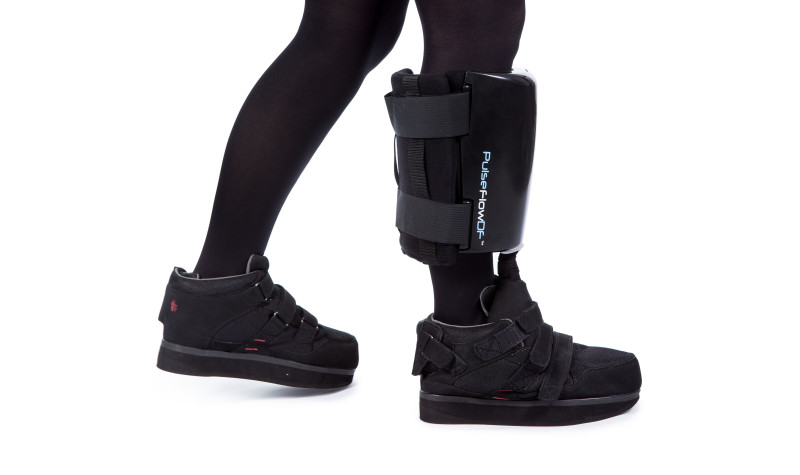
Les Lindsay (CEO): PulseFlow Technologies (PFT) is the trading name for The Diabetic Boot Company Ltd (DBC). DBC was founded in February 2010 after we were granted a patent in December 2009. We have been funded since inception, first by our Directors, then by Business Angels and now via crowd funding plus one or two institutional investors. In total we have had around £3.8 million in investment, and we are now gearing up to begin mass production of our product, PulseFlowDF. We have been developing our product since inception in close liaison with global experts in the diabetic limb salvage arena, and we have now been cleared to begin selling in most of our target markets. We have developed PulseFlowDF to be an active wound healing system that is a Class 2 (IIa) medical device to help diabetic foot ulcers (DFUs) heal faster and more reliably.
Diabetes is a growing problem in every country in the world. Around 5-15% of diabetics will have a foot ulcer on any given day, and 25% of diabetics get an ulcer at some stage in their life. Around 20% of these ulcers won’t heal, and lead to some part of the foot or leg being amputated. Our product will get more ulcers to heal more quickly, and will prevent some patients suffering an amputation, thereby becoming disabled. The five-year survival rate for someone post amputation is worse than for breast cancer victims. These figures lead to huge amounts of money being spent each year on treating DFUs, and in the UK, we spend over £600m annually but still amputate around 135 extremities per week. We have around 3 million DFUs in the USA, and that represents our biggest market opportunity.We have opened an office in the US, and it is now registered to begin operating under license to sell PulseFlowDF as durable medical equipment under an existing reimbursement code.
JF: It is pretty shocking to think that every 20 seconds someone in the world loses a limb to diabetes. Studies show that 50% of those people die within two years. Can you put this into context for us in terms of the global market opportunity for PulseFlow?
LL: It is indeed shocking, James, and I have to say that it is an under-recognised threat for a lot of people with diabetes. In the early days, when I was pitching to potential investors, diabetes was understood to be a growth market, but diabetic foot health was not seen as an area of huge need. The first few business angels who took a risk on our company, usually had some insight into the seriousness of the condition due to a friend or family member suffering from a foot ulcer that took ages to heal, if ever. One investor lost his father to double amputation consequences following non-healing ulcers. Our job is to give these highly motivated limb salvage clinicians another tool to try and get the ulcer to close over before infection gets into the bone. Infected foot bones mean either an expensive hospital stay for IV antibiotics, or straight to amputation.
We are offering a non-surgical intervention at a cost most economies in the developed world can afford. We also have some plans to assist those economies who cannot afford “western” prices. Certain target markets like the Middle East have huge numbers of patients relative to their populations; an example of this would be Saudi Arabia where a population circa 25m, may have more than 300,000 DFUs on any one day. Overall, using conservative figures, our addressable market is circa $1.7 billion dollars just for PulseFlowDF, let alone the line extensions we have in the planning stage.
JF: This is clearly a huge problem for hard-pressed western healthcare systems. Apparently, each $1 invested in care by a podiatrist results in between $27 to $51 of healthcare savings. Surely this is a no-brainer for organisations such as the NHS? What kind of reception have you had for the product thus far?
LL: The NHS clinicians like the idea, but take-up has been slow, even though we managed to get the product on to the NHS Supply Chain catalogue. I don’t think this is unique to our product though. Many of our investors were actually relieved that we were not forecasting huge early sales to our home market, as they had seen so many other portfolio companies struggle to get a foothold in an organisation that positively resists rather than embraces new technologies. We are working with the folks at NICE in a measured way to have our rapidly building clinical evidence put through an impartial review to see where they feel it could be deployed by a struggling NHS to reduce amputation rates.
Our business plan has always shown that our most rapid sales growth will come from export sales, and the feedback we have from Germany, Austria, New Zealand, and Australian distribution partners is far more go-ahead acceptance by clinicians. The response from our main target market, the USA, has been so positive that we have set up our own operation there, and we are forecasting exceptionally high take-up by an open-to-technology marketplace and clinician group. We will also be harnessing the power of lobby groups to make things happen, and social media will play an important part in our success story. Every hour of every day, some concerned daughter types “how do I save my father’s toes from amputation”; we need to give those people easy access to our informative website so they can discuss the applicability of PulseFlowDF to assist their parent’s condition.
JF: What makes the Pulseboot unique and how is the firm’s IP protected from potential competitors? Are there any other similar products or substitutes on the market?
LL: PulseFlowDF is absolutely unique in many ways, but there are 4 main USPs to discuss:
- It takes away pressure shear and friction forces from the wound bed to allow the wound to heal faster.
- Normal pressure-relieving boots (usually made from plastic) actually reduce the amount of blood flowing through the diabetic foot, which may already have limited blood flows due to narrowing of the arteries. PulseFlowDF has an active pumping mechanism to increase blood flows back to the heart against gravity, with subsequent increases in oxygenated blood flowing down to the foot. More oxygen in a wound equals more rapid healing.
- The diabetic patients are renowned for not always heeding doctors’ advice. To assist clinicians understand what the patient is actually doing to follow guidance, we have built in software that shows the clinician how many hours the boot has been worn pumping blood. In early feedback from clinicians, they say that this one feature has enabled them to have much more, shall we say, interactive discussions with their non compliant patients, with an observed rise in wear time after such conversations.
- Added Value: in early discussions with limb salvage experts, it became apparent that whilst they don’t mind paying for a therapy, what they really desired was an extension of the “ulcer free period”. Our US Advisor, Dr David Armstrong, likens DFUs to cancer; you can never 100% say you have cured a cancer, but you have sent it into a remission period. DFUs often reoccur at the same site within weeks of being healed, because the patient goes back to wearing “normal” shoes with no pressure relief. PulseFlowDF is unique in that after therapy, the pumping part of the medical device is removed, but the patient keeps the footwear to offer continued protection to the foot whilst the ulcer’s newly grown skin is allowed to “toughen up” and become more resilient to breakdown. The PulseFlowDF is single patient use, so we don’t want potentially infected footwear coming back to us anyway. The therapy part is also single patient use, and cannot be recycled for further patients.
JF: Presumably the product has patent protection?
LL: We do have an increasingly robust IP portfolio, James. Our initial grant in December 2009 was pretty wide-ranging, but based on the experience I had with KCI Medical, I have made sure that we keep adding nuances to the IP we have to keep our knowledge base protected. We are now starting to think about the R&D programme for PulseFlowDF V2, but we made sure we had extra claims filed before we started to discuss it with potential development partners. Whilst I accept the absolute need for IP protection, I am also actively building barriers to entry to mitigate the potential disruption should any company manage to get round the IP.
JF: Pulse-Flow recently became one of the UK’s highest value crowd funded companies to date after raising £1.75 million via Syndicate room in a fundraising led by our very own Jim Mellon. What factors led you to go down the crowdfunding route? Is crowdfunding is now a serious alternative to conventional equity and debt financing?
LL: Crowd funding was something of an experiment for us, and whilst it worked out in the last round, I am not sure we will actively pursue it in the current round. The reason being is that numerically, it expanded our investor base by a huge amount, and it did give us a challenge completing all the paperwork in a timely fashion post transaction.
JF: Are there any upcoming catalysts/milestones for PulseFlow that we should be on the lookout for?
LL: We gained the green light, FDA 510k clearance, in December 2015, which was a huge milestone for us. The next biggest challenge will be to get final sign-off on a US reimbursement rate at a high enough level. I would guess we are now 85% through that process, so a very significant short-term milestone will be to have that rate approved by CMS/Medicare before May 2016. That would mean we had clearance to market and a reimbursement rate to go forth and multiply in the DFU plague present in the USA. After the USA, with existing regulatory affairs clearances (CE mark etc.) we look for significant revenues coming from Distribution partners we have already signed up with. All of the above is dependent upon ramping up production capacity in the short term, and we are probably 90% complete on that, awaiting formal contracts being signed up. We will commission large scale cost effectiveness / health economics studies in 2016, and they should report by 2017.
JF: We’ll certainly be keeping an eye out for those developments, Les. Thanks for your time today. And good luck with what is clearly a product that has the potential to do so much good!
LL: Thanks, James!
P.S. You’ll be able to hear more from this exciting company at this year’s Master Investor Show in Islington on 23rd April. Click HERE to book your complimentary tickets.
Comments (0)