A Whole New Angle on Cancer Diagnosis
An Interview with Andrew Newland, CEO of Angle (AIM:AGL) Plc
James Faulkner: Hi Andrew. The last time we met Angle had several different projects in development, but now the company is focused solely on commercialising its patented Parsortix liquid biopsy system, which has potential applications in the diagnosis and treatment of certain cancers. Please take us through the recent transformation of Angle and explain the reasons behind it.
Andrew Newland: ANGLE has specialised in identifying and developing high growth patent backed businesses. A few years ago we realised how big the liquid biopsy opportunity and our patent protected product based Parsortix business could be and since then we have divested our other businesses and focused all our resources on Parsortix.
 JF: In layman’s terms, how does Parsortix work?
JF: In layman’s terms, how does Parsortix work?
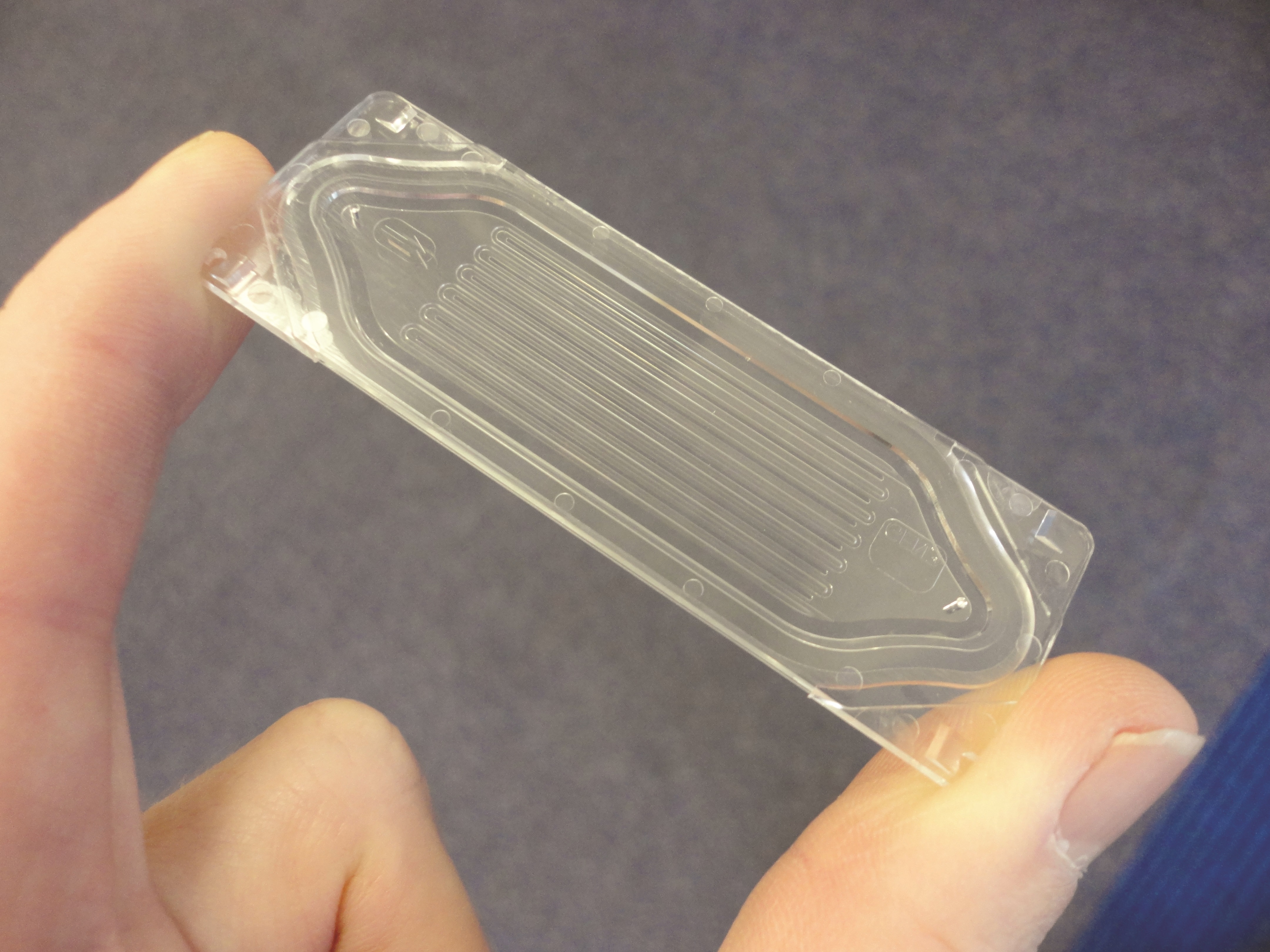
AN: Very simple. Parsortix isolates rare cells in blood based on their larger size and lack of compressibility meaning we can find 1 cell in a billion and also harvest (recover) them. We have a fully developed patented microfluidic system able to do this.
It works for various different types of rare cells but our main focus is cancer.
JF: What are the key advantages of the IP behind Parsortix and how is that IP protected against competitors?
AN: We have been two granted US patents, and European, Chinese, Canadian and Australian patents, and we have patents being progressed worldwide that give us a monopoly over our microfluidic solution through the use of steps to capture particles in liquid and in this case cancer cells in blood. The step based approach is very simple with the result that we have a low cost base approach.
JF: What is the potential size of the market opportunity for Parsortix? What kind of market share does Angle hope to achieve with this product?
AN: Our estimate is that the market is potentially worth £8 billion per annum to ANGLE. Clearly that is a huge number. The crucial issue is delivering this business plan. So far we have a sales plan in place for research use addressing £250 million per annum and studies in progress in relation to clinical applications the first being ovarian cancer worth £300 million per annum. We have also had great results which we will most likely develop shortly in prostate cancer worth £3 billion per annum and breast cancer (just announced this week!) worth at least £1 billion per annum. ANGLE aims to be a leading player in this market and is developing clinical applications where we are clearly differentiated.
 JF: What does the route-map to commercialisation look like and how far along is Parsortix in the commercialisation process?
JF: What does the route-map to commercialisation look like and how far along is Parsortix in the commercialisation process?
AN: In order to become a new standard of care we need patient data and regulatory authorisation. We have a CE Mark in Europe and we have been working for two years with the FDA and are hoping to be the first company ever authorised by the FDA for harvesting cancer cells from patient’s blood for analysis. Liquid biopsies will greatly improve patient treatment and outcomes but will also save a lot of healthcare money.
We have patient studies already in progress for ovarian cancer which we hope will complete by the end of 2016.
JF: Clearly, securing FDA approval will be a major milestone for Parsortix. How advanced are your discussions with the FDA and when can we realistically expect final authorisation in the US?
AN: Well advanced, two years’ work in fact! We are working closely with the FDA to provide a fully validated liquid biopsy, which enables a simple blood test to be used in place of invasive tissue biopsies. We are currently working on providing additional analytical (internal tests) and clinical (on patients) studies being required to complete the authorisation for metastatic breast cancer. While we aim to make substantial progress this year it is not possible to determine when we might receive final authorisation as the FDA will need time to review the data and could raise additional questions.
JF: Angle held cash balances of £5.8 million as at 31st October 2015 and recently received £0.7 million from the sale of Geomerics. Do you expect the company’s current financial resources to be sufficient to take Parsortix through commercialisation, or will there be a fundraising at some point?
AN: We are funded for the ovarian cancer application, building research use sales and FDA. This is a huge market opportunity and if we want to pursue other applications such as prostate and breast cancer we will need additional funds.
JF: Does Parsortix have any other potential applications beyond the cancer market?
AN: Yes, we are focused on cancer cells in blood at the moment but there are other cells of great importance. Originally we developed the Parsortix system to capture fetal cells in maternal blood. Perhaps one day we will return to that.
JF: What milestones should investors look out for in the near future?
AN: There has been a lot of positive news recently, for example in prostate and breast cancers, and we expect our KOLs (Key Opinion Leader cancer centres) to keep producing game changing information and additional clinical applications.
We have very important trials in ovarian cancer and metastatic breast cancer for the FDA in progress and we expect to have similar trials in prostate and breast cancers shortly.
JF: Angle will be presenting at the Master Investor Show in Islington on 23rd April. You also have a speaking slot on the Rising Stars Stage. Where can readers find you on the day?
AN: Yes we will be presenting on the Rising Stars Stage at 13:50. We also have an exhibition stand with our Parsortix system on display and scientists available to discuss the relevance of what we are doing.
Comments (0)